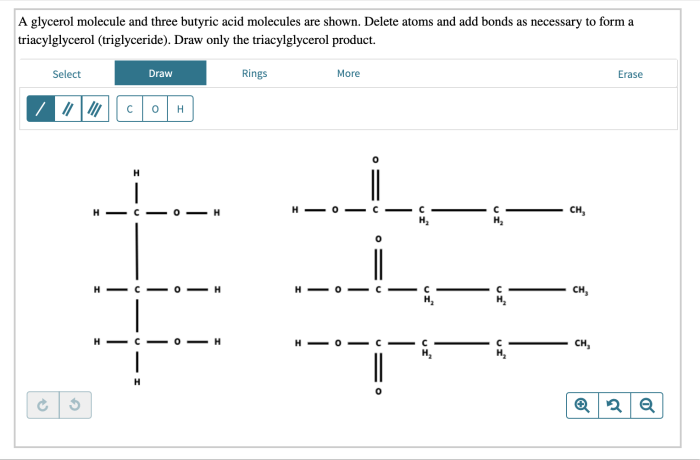
In the realm of organic chemistry, a captivating journey unfolds as we delve into the intriguing interplay between a glycerol molecule and three butyric. This encounter, known as esterification, gives rise to glyceryl tributyrate, a versatile compound with a myriad of applications.
Our exploration begins with an in-depth examination of the structure and properties of glycerol and butyric acid. We will unravel the intricate process of esterification, detailing the conditions and steps involved in this chemical transformation. Finally, we will uncover the remarkable properties and diverse uses of glyceryl tributyrate.
Glycerol Molecule
Glycerol is a simple polyol compound with the formula C3H8O3. It is a colorless, viscous liquid that is soluble in water and alcohol. Glycerol is a trihydroxy alcohol, meaning that it has three hydroxyl (-OH) groups attached to a central carbon atom.
Properties of a Glycerol Molecule
- Glycerol is a viscous liquid with a density of 1.26 g/cm3.
- It is soluble in water and alcohol, but insoluble in nonpolar solvents such as ether.
- Glycerol has a sweet taste and is non-toxic.
- It is a humectant, meaning that it can absorb and retain water from the air.
Examples of How a Glycerol Molecule Is Used, A glycerol molecule and three butyric
- Glycerol is used in the manufacture of soaps, detergents, and other personal care products.
- It is also used in the food industry as a humectant and sweetener.
- Glycerol is used in the pharmaceutical industry as a solvent and excipient.
Butyric Acid
Butyric acid is a short-chain fatty acid with the formula CH3CH2CH2COOH. It is a colorless liquid with a strong, unpleasant odor. Butyric acid is found in butter, cheese, and other dairy products. It is also produced by the fermentation of carbohydrates by certain bacteria.
Chemical Formula of Butyric Acid
The chemical formula of butyric acid is CH3CH2CH2COOH.
Physical and Chemical Properties of Butyric Acid
- Butyric acid is a colorless liquid with a strong, unpleasant odor.
- It is soluble in water and alcohol, but insoluble in nonpolar solvents such as ether.
- Butyric acid has a boiling point of 163.5 °C and a melting point of -5.5 °C.
- It is a weak acid with a pKa of 4.82.
Examples of How Butyric Acid Is Used
- Butyric acid is used in the manufacture of butter, cheese, and other dairy products.
- It is also used in the food industry as a flavoring agent.
- Butyric acid is used in the pharmaceutical industry as a solvent and excipient.
Esterification of Glycerol and Butyric Acid
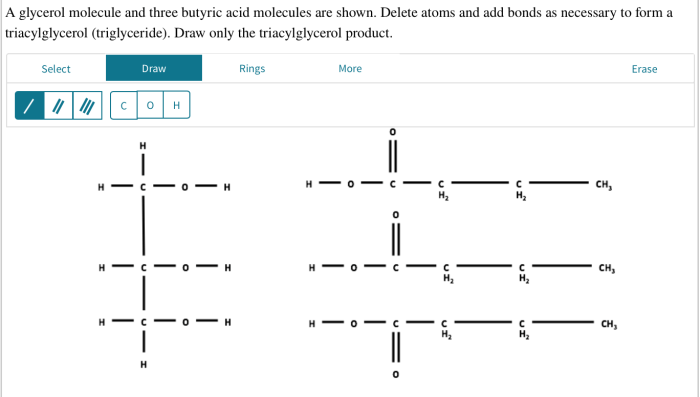
Esterification is a chemical reaction in which an alcohol and a carboxylic acid react to form an ester. The esterification of glycerol and butyric acid produces glyceryl tributyrate, which is a triglyceride.
Conditions Necessary for the Esterification of Glycerol and Butyric Acid
- The reaction must be carried out in the presence of an acid catalyst, such as sulfuric acid or hydrochloric acid.
- The reaction must be heated to a temperature of around 100 °C.
Step-by-Step Procedure for the Esterification of Glycerol and Butyric Acid
- Add glycerol and butyric acid to a round-bottomed flask.
- Add a few drops of sulfuric acid to the flask.
- Heat the flask to a temperature of around 100 °C.
- Stir the reaction mixture for several hours.
- Allow the reaction mixture to cool.
- Pour the reaction mixture into a separatory funnel.
- Separate the organic layer from the aqueous layer.
- Wash the organic layer with water.
- Dry the organic layer over anhydrous sodium sulfate.
- Filter the organic layer.
- Distill the organic layer to obtain glyceryl tributyrate.
Properties of Glyceryl Tributyrate: A Glycerol Molecule And Three Butyric
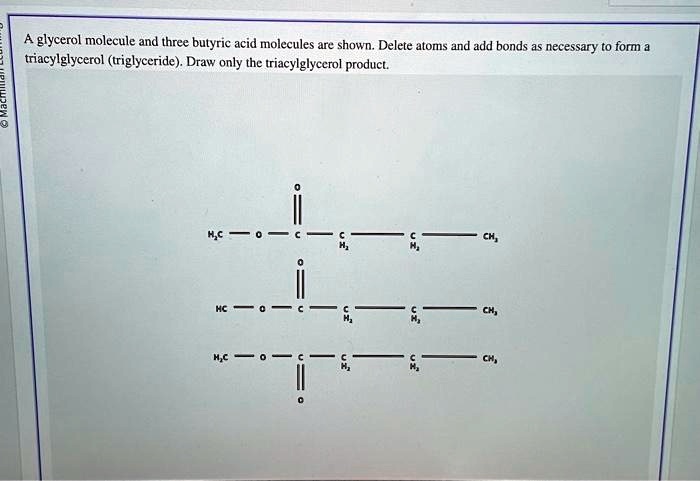
Glyceryl tributyrate is a triglyceride that is composed of three butyric acid molecules attached to a glycerol molecule. It is a colorless liquid with a mild odor. Glyceryl tributyrate is insoluble in water, but soluble in organic solvents such as ether and alcohol.
Physical and Chemical Properties of Glyceryl Tributyrate
- Glyceryl tributyrate is a colorless liquid with a mild odor.
- It is insoluble in water, but soluble in organic solvents such as ether and alcohol.
- Glyceryl tributyrate has a boiling point of 256 °C and a melting point of -7.5 °C.
- It is a non-toxic compound.
Applications of Glyceryl Tributyrate
- Glyceryl tributyrate is used in the food industry as a flavoring agent.
- It is also used in the pharmaceutical industry as a solvent and excipient.
- Glyceryl tributyrate is used in the cosmetics industry as an emollient.
Examples of Products That Contain Glyceryl Tributyrate
- Butter
- Cheese
- Ice cream
- Margarine
- Salad dressings
FAQs
What is the structure of a glycerol molecule?
A glycerol molecule consists of a central carbon atom bonded to three hydroxyl groups (-OH).
What is the chemical formula of butyric acid?
The chemical formula of butyric acid is CH3CH2CH2COOH.
What are the applications of glyceryl tributyrate?
Glyceryl tributyrate is used as a food additive, a plasticizer, and in the production of pharmaceuticals and cosmetics.